
PRODUCT INFORMATION

CovClear COVID-19 Rapid Antigen Self Test
The CovClear Rapid Antigen test is a lateral flow assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2 in nasal swab specimens directly collected.
Features:
- Simple to use with rapid results in minutes
- High sensitivity, specificity and accuracy
- Safety advantage with sealed vial protecting against live virus
- No reader or cassette required, reducing cost and waste
- Researched, developed & manufactured in the USA
Specification:
- Sample Type: Nasal swab
- Detection Time: 20 minutes
- Sensitivity/PPA: 95.5 %
- Specificity/NPA: 100 %
- Manufactured in the United States
- Interim order #330172
- Materials required but not provided: a pair of gloves and timer
Each Box Contains:
| 2-pack | 25-pack | 50-pack |
| 2 Lateral flow assay strips | 25 Lateral flow assay strips | 50 Lateral flow assay strips |
| 2 Chase buffer ampules | 25 Chase buffer ampules | 50 Chase buffer ampules |
| 2 Vials | 25 Vials | 50 Vials |
| 2 Locking caps | 25 Locking caps | 50 Locking caps |
| 2 Individually wrapped swabs | 25 Individually wrapped swabs | 50 Individually wrapped swabs |
| 2 Instructions for use | 25 Instructions for use | 50 Instructions for use |
| 2 Quick reference guides | 25 Quick reference guides | 50 Quick reference guides |
Interpretation of Results
Sample Collection
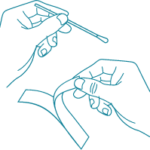
1) Remove a swab from the pouch.
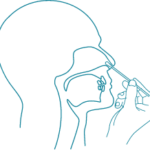
2) Place the dry swab into one nostril until it reaches resistance.

3) Slowly rotate the swab 7 times over the surface inside the nostril.
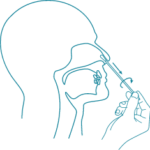
4) Slowly remove the swab from the nostril while still rotating it. Repeat steps 2-4 on other nostril.
Test Instructions
1) Remove a swab from the pouch.
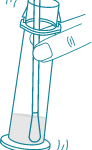
2) Place the dry swab into one nostril until it reaches resistance.

3) Slowly rotate the swab 7 times over the surface inside the nostril.
NOTE: DO NOT place the cap on the vial. DO NOT remove the swab.

4) Slowly remove the swab from the nostril while still rotating it. Repeat steps 2-4 on other nostril.
NOTE: DO NOT remove the swab.

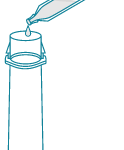
5) Place new test strip, arrow pointing down, into vial with the swab.
NOTE: Touch test strip on the colored end only.

6) Place cap securely onto the vial with the test strip and swab inside.
NOTE: Cap will permanently lock in place. DO NOT tip vial over as this will invalidate the test.
READ RESULTS AFTER 20 MINUTES.
Interpretation of Results
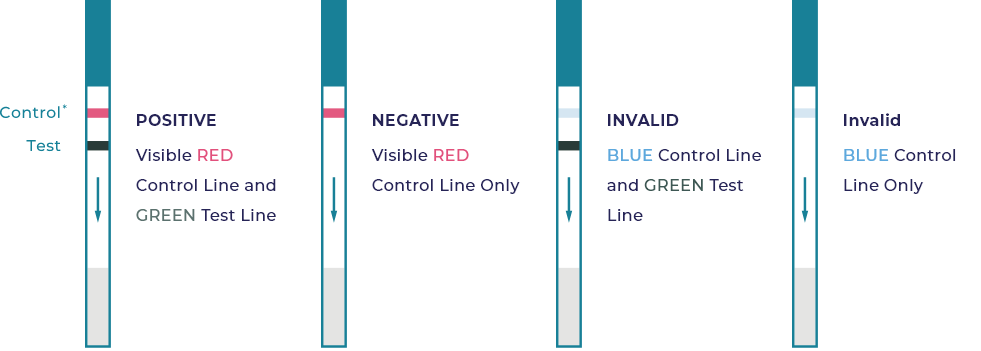
Limitations
- Failure to follow the instructions for use may adversely affect test performance and/or invalidate the test result.
- Results from antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2
infection or to determine infection status. - The detection of SARS-CoV-2 nucleocapsid antigen is dependent upon proper specimen collection, handling, storage, and preparation. Failure to observe proper procedures in any one of these steps can lead to incorrect results.
- Results from the device should be correlated with the clinical history, epidemiological data and other data available.
- False-negative results may occur if the concentration of the target antigen in the clinical specimen is below the detection limits of the device.
- There is a higher chance of false negative results with home use tests than with laboratory-based molecular tests. This means that there is a higher chance this test will give you a negative result when you have COVID-19.
- The performance of this device has not been assessed in a population vaccinated against COVID-19.


