
PRODUCT INFORMATION
One provider, multiple rapid-testing solutions
Inquire now for FDA/EUA Approved COVID-19 test kits.
FDA/EUA Authorized Products
Healgen
COVID-19 Antigen Rapid Test

Each Kit Contains:
- 20 Test Cassettes
- 20 Sterile Swabs
- 20 Pre-filled buffer tubes with flip-top
- 2 Tube Holders
- 1 Instructions for Use
- 1 Quick Reference Guide
Specification:
- Relative sensitivity: 85.4%, Relative specificity: 99.7%
- Sample Type: Nasal
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
Key Features:
- FDA cleared & CLIA waived
- Non-Invasive
- Simple to use
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 20 tests per kit
Resources
INDICAID
COVID-19 Antigen Rapid Test

Each Kit Contains:
- 2/25 Test cassettes
- 2/25 Sterile swabs
- 2/25 Extraction buffer vials
- 1 Package insert and quick reference guide
Specification:
- Relative sensitivity: 84.4%, Relative specificity: 97%
- Sample Type: Anterior Nasal swabs
- Detection Method: Colloidal Gold
- Detection Time: 20 minutes
Key Features:
- FDA EUA Approved
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 2/25 tests per kit
Healgen
COVID-19/Flu A&B Antigen Test

Each Kit Contains:
- 1/2/4 Sealed Test Cassette(s)
- 1/2/4 Sterile Nasal Swab(s)
- 1/2/4 Pre-filled Extraction Tube(s)
- 1/2/4 Extraction Tube Tip(s)
- 1 Tube Holder
- 1 Quick Reference Instructions (QRl)
Specification:
- COVID-19 – Sensitivity 92.0%, Specificity 99.0%
- Flu A – Sensitivity 92.5%, Specificity 99.9%
- Flu B – Sensitivity 90.5%, Specificity 99.9%
- Sample Type: Nasal Swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
Key Features:
- Over The Counter Use
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 and Influenza testing
- Cost-efficient
Packaging Configuration:
- 1/2/4 tests per kit
Resources
CorDx
COVID-19 Antigen Test
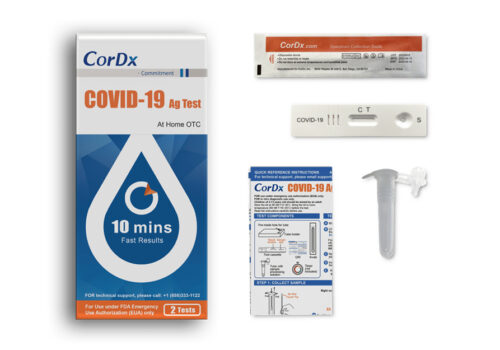
Each Kit Contains:
- 2 Single-use test devices
- 2 Single-use tubes with sample processing solution
- 2 Single-use specimen sampling swabs
- 1 Quick reference instructions (QRI)
Specification:
- Relative sensitivity: 89.7%, Relative specificity: 99.7%
- Sample Type: Anterior Nasal
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
Key Features:
- FDA EUA Approved
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 2 tests per kit
STATUS™
COVID-19/Flu A&B Rapid Antigen Test
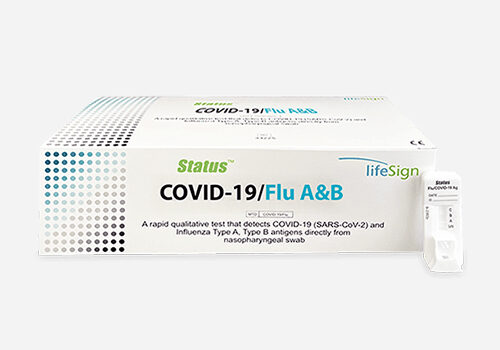
Each Kit Contains:
- 25 Test cassettes
- 25 Sterile swabs
- 25 Extraction buffer vials
- 1 Positive Control Swab: Influenza A, B, and SARS-CoV-2 antigen
- 1 Negative Control Swab
- 1 Package Insert /Instructions for use
- 1 Quick Reference Instructions
Specification:
- COVID-19 – Sensitivity 93.9%, Specificity 100%
- Flu A – Sensitivity 91.4%, Specificity 95.7%
- Flu B – Sensitivity 87.6%, Specificity 95.9%
- Sample Type: Nasopharyngeal or Anterior Nasal Swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
Key Features:
- FDA EUA Approved
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 and Influenza testing
- Cost-efficient
Packaging Configuration:
- 25 tests per kit


