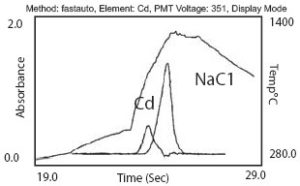
Scientific and technological advancements are forever pressing forward, providing society with innovative solutions to age-old dilemmas. Atomic absorption spectroscopy is one such advancement that has yielded numerous benefits in various industries. Though this process dates back to the mid-19th century, recent advancements in technology and the use of automated workstations now enable scientists to perform these processes with increased efficiency and reliability. Below is a brief explanation of the history and purpose of this process—including some of the more common atomic absorption techniques and applications in practice today.
What is atomic absorption spectroscopy?
Atomic absorption spectroscopy, often abbreviated AAS, is the process which tests the concentration of gas-phase atoms within a given sample. The concentration of these atoms is determined by testing the amount of light absorbed by the free ions within the sample. By exposing a sample to light at a specific wavelength and tracking how much of that light is absorbed by the sample, scientists are not only able to determine an element’s presence within a sample, but also that element’s concentration. Atomic absorption spectroscopy can detect roughly 70 different elements and can be utilized in both solid and liquid samples; though, the experimentation of solid samples does require additional processes. Atomic absorption spectroscopy is utilized across many industries and is instrumental in the detection of metals within a sample. As such, this process is commonly utilized in pharmacology, archaeology, manufacturing, mining, and forensics.
The history of atomic absorption spectroscopy
The analysis of atomic absorption spectroscopy began in the mid-19th century with studies by Gustav Kirchhoff and Robert Bunsen. Utilizing the knowledge of their predecessor Joseph von Fraunhofer and a new flame source devised by Bunsen himself—the now popular Bunsen burner—the pair began to experiment with the spectra of various chemical compounds. Throughout the course of their studies, Kirchhoff and Bunsen discovered that each chemical element possesses a unique spectral pattern that reacts differently when exposed to various light wavelengths. Using these findings, they then demonstrated the many ways spectroscopy could be utilized in trace chemical analysis and the discovery of previously unknown chemical elements. Their experiments identified the presence of several elements within natural compounds and gave way to atomic absorptions spectroscopy’s use in agriculture and environmental sciences. The findings of these experiments also eventually gave way to subsequent studies which established a link between absorption and emission lines, enabling scientists to trace solar absorption lines to specific elements based solely on spectra. Kirchhoff studied the link between absorption and emission even further and eventually developed what is now known as Kirchhoff’s Law of Thermal Radiation.
Techniques of atomic absorption spectroscopy
Whereas atomic absorption spectroscopy is the study of how light and energy interact with matter, atomic absorption spectrometry refers to the techniques and methodology used to apply this study to real-world practices. To reiterate the above statement, atomic absorption spectrometry can be conducted with either solid or liquid samples. However, because this process requires atoms to be in a gaseous state, the solid or liquid sample must be vaporized and the analyte atoms within the sample must be atomized. This can be done using one of two main methods listed below.
Flame atomizers
Flame atomizers, frequently abbreviated FAAS, are the oldest and most commonly used atomizers in atomic absorption spectroscopy. FAAS is most commonly used to test liquid samples or solid samples which have been dissolved within a liquid. Using this atomization process, a sample is first evaporated, leaving behind only the sample’s dry nano-particles. These solid particles are then vaporized and converted into gaseous molecules, which can then be dissociated into free atoms. Finally, the atoms are converted into gaseous ions and can be exposed to a small flame, which can reach extremely high temperatures. It is during this step in the process that the sample is finally exposed to a radiation beam and the signal of absorbed electromagnetic radiation is measured.
Electrothermal atomizers
Electrothermal atomizers are sometimes also referred to as graphite furnace atomizers, as they utilize a graphite tube to heat samples, rather than a flame. Unlike flame atomization, which transforms the sample solution into an aerosol and mixes it with flame gases, this technique allows liquid, solid, and gaseous samples to be analyzed directly. Electrothermal/graphite furnace atomizers, sometimes abbreviated ETAAS or GFAAS, deliver signals in a discontinuous mode. By contrast, flame atomizers deliver signals in a continuous fashion. ETAAS/GFAAS also minimize interference problems and can determine a wide variety of elements for most matrices.
Applications
As we’ve stated, atomic absorption spectroscopy is utilized in a wide variety of industries and areas of scientific study. Some of the more common applications for this technique are listed below.
Agriculture
Atomic absorption spectroscopy is frequently utilized in agriculture and the study of environmental sciences. Atomic absorption spectroscopy, as well as atomic fluorescence spectroscopy—which analyzes the light emitted from a sample rather than the light absorbed—are frequently used in various fields of agricultural study. Typically, they’re used to identify and analyze the presence of potentially harmful elements in the environment.
One common use for these methods is the analysis of soil samples and the effect the quality of the soil will have on the overall rate of food production in a certain area. Soil samples that contain high levels of phosphorous and nitrogen often yield higher production volume and produce healthier crops. Atomic absorption and atomic fluorescence spectroscopy can be used to determine the presence of these elements and the quantities in which they appear. These methods can also be used to detect trace amounts of harmful chemicals, such as rhodium, in water samples.
Forensics
Atomic absorption spectroscopy has been utilized in the study of forensic sciences for many years. Using this technology, forensic scientists can perform in-depth analysis of blood samples, brain and muscle tissue, and gunshot powder residue. This technology has vastly improved the accuracy of toxicology reports in cases of metal poisoning. Common causes of metal poisoning, such as mercury and lead, are easily detectable using this technology and can be identified even in trace amounts.
To learn more about Atomic Absorption Spectrometers, click here.