Two of the most widely used vaccines during the current COVID-19 pandemic are the mRNA vaccines developed by BioNTech/Pfizer and Moderna. Here’s a look at some of the most common questions regarding these vaccines.
How do these vaccines differ from conventional vaccines?
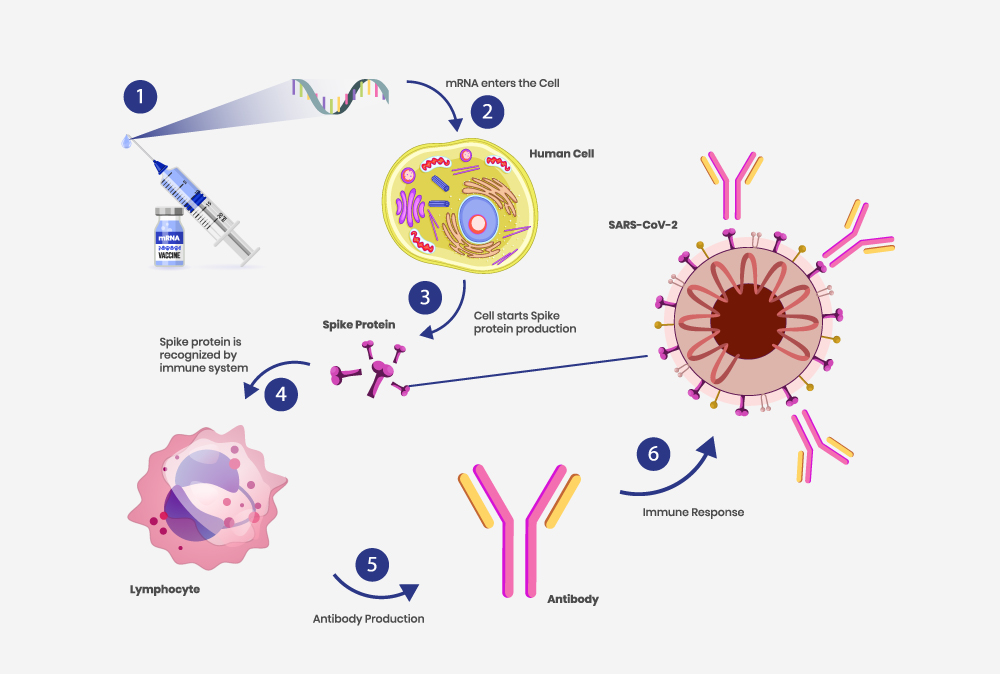
mRNA vaccines are based on a newer technology and unlike traditional vaccines, do not use live/inactivated virus to trigger an immune response. Instead, they use modified mRNA to send instructions to our cells to make proteins, specifically portions of the S (spike) protein, which then triggers an immune response. Once triggered, our body makes antibodies. These antibodies help us fight the infection if the real virus does enter your body in the future.
Were these vaccines rushed?
No, the mRNA vaccines have not been rushed. In fact, mRNA vaccine development has been based on decades of scientific research. These vaccines were created, clinically evaluated and authorized for emergency use around the world in under a year. Despite the fast timeline, both the vaccines went through the appropriate evaluation process including clinical trials, just like all other vaccines before. Health authorities continue to monitor them closely for safety and efficacy.
The FDA has granted full approval to the Pfizer/BioNTech COVID-19 vaccine. How is this different from emergency use authorization (EUA)?
In August 2021 the FDA granted full approval to the Pfizer/BioNTech COVID-19 mRNA vaccine for use in people aged 16 years and older. The first such approval for COVID-19 vaccines.
The transition from EUA status to full approval was based on additional data showing that the vaccine met certain safety, effectiveness, and manufacturing quality standards. For example, data from a clinical study involving 44,000 participants, half of whom received two doses of the Pfizer/BioNTech vaccine, was analyzed. The participants were followed up for 6 months. To ensure quality, FDA also conducted inspections of the Pfizer/BioNTech’s manufacturing facilities. Safety data continues to be collected and monitored. In Canada, health care providers are required to report adverse events following immunization to their local public health authorities, who in turn report them to the Public Health Agency of Canada.
What are breakthrough infections, and why do they happen?
A breakthrough infection occurs when a fully vaccinated individual gets infected with COVID-19. A small number of breakthrough infections was always expected since none of the vaccines were a 100% effective in clinical trials. In its Morbidity and Mortality Weekly Report (MMWR), CDC recently reported that there were 10,262 breakthrough infections through the end of April 2021. By that point, more than 100 million Americans had received the COVID-19 vaccine.
While a vast majority of these infections were either asymptomatic or mild to moderate, around 1000 patients (10%) were hospitalized. Of these,160 (2%) died although many of the deaths were due to causes unrelated to COVID-19.
Do I need a third dose of the COVID-19 vaccine?
In August 2021, the FDA issued EUAs to both the Pfizer-BioNTech and the Moderna COVID-19 vaccines for an additional dose for people who are immunocompromised. In early September, Canada’s national advisory body on vaccines recommended giving third dose of COVID-19 vaccines to immunocompromised individuals. No decision has been made on the third dose for the general population.
Immunocompromised individuals have a weakened immune system and may not be able to produce a strong enough immune response to the standard, two-dose COVID mRNA vaccine regimen. This not only makes them more susceptible to a breakthrough infection, but also increases the risk of more severe symptoms if they do become infected. A third dose of the vaccine may enhance their immune response. It is recommended that anyone with a weakened immune system should continue to wear masks and avoid crowded and poorly ventilated indoor spaces.
What about the delta variant?
The delta (B.1.617.2) variant that first emerged in India earlier this year is now the most common COVID-19 variant in both US and Canada. It is nearly twice as contagious as earlier variants and might cause more severe illness.
Several different studies have been carried out over the past few months regarding the efficacy of both the mRNA vaccines against variants, particularly the delta variant. The vaccines still appear to provide strong protection against severe infections with COVID-19, although almost all the studies also show decrease in effectiveness ranging from about 50-95%.
What is the future for mRNA vaccines?
mRNA technology has been developing, albeit slowly, over the past few decades and researchers have applied it not just in the development of novel vaccines but also for therapeutics for cancer and gene therapy. mRNA technology is currently being applied to the emerging field of cancer immunotherapy. Moderna, for example, is in the process of creating an mRNA-based personalized cancer vaccine.
In terms of vaccine technology, researchers have been working on developing mRNA vaccines for other infectious diseases long before the COVID-19 pandemic hit. Studies in animal models have shown that mRNA vaccines elicited potent immune response against viruses like influenza, Zika and rabies. Research into developing vaccines for the dengue virus as well as malaria is gaining ground. BioNTech recently announced development of the first vaccine for malaria based on mRNA technology, with clinical testing set to begin next year and Moderna recently announced human trials for an m-RNA based HIV vaccine.