PRODUCT INFORMATION
Cardiac safety is a critical aspect of drug development, and issues related to cardiac function and proarrhythmic risk can lead to clinical trial delays, drug development program discontinuation or even drug removal from the market. To address these challenges, Aurora Biomed offers an integrative approach using various functional assays to assess drug-induced effects on the heart at multiple levels.
Our approach combines industry-standard protocols to provide comprehensive insights into the cardiac safety profile of compounds. By employing a range of functional assays, we can evaluate potential drug-induced proarrhythmia and cardiac dysfunction.
By combining ion channel screening with action potential (AP) recordings and contractility measurements, we can provide a comprehensive assessment of the cardiac safety profile of compounds, helping to identify potential issues early in the drug development process. Our integrative approach enables us to offer tailored solutions to meet the specific needs of our clients, ultimately leading to safer and more effective therapies
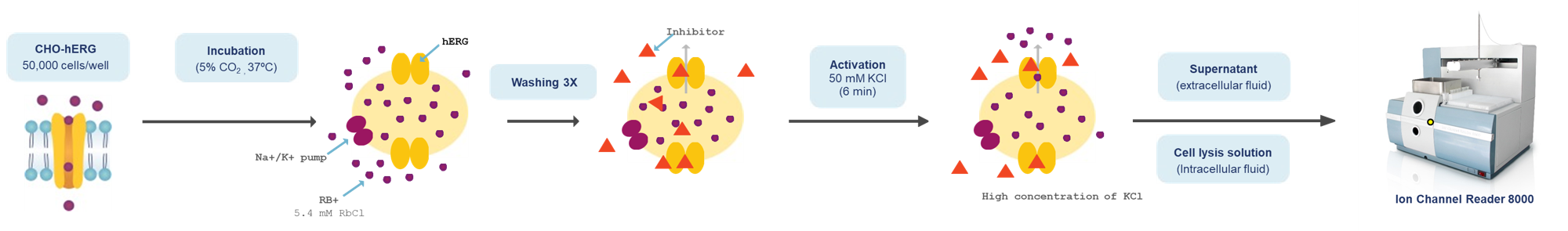
Combining high throughput ion channel screening with the gold standard manual patch clamp technique. We apply protocols and best practice as described in the International Council for Harmonisation (ICH) E14/S7B Q&As (February 2022) and overexpressing cell lines to determine drug potency (IC50 and Hill coefficient) on the hERG channel.
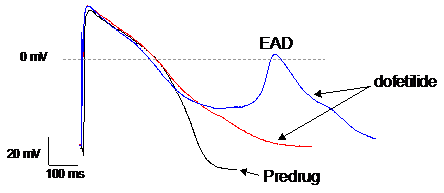
We use manual patch clamp to detect drug-induced changes in action potential morphology recorded from human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM). Changes in the various phases of the action potential reflect the integrated drug effects on one or multiple ion channels. Abnormal repolarization under the form of early after depolarizations (EADs) or delayed after depolarizations (DADs) is an indication of proarrhythmic liability.
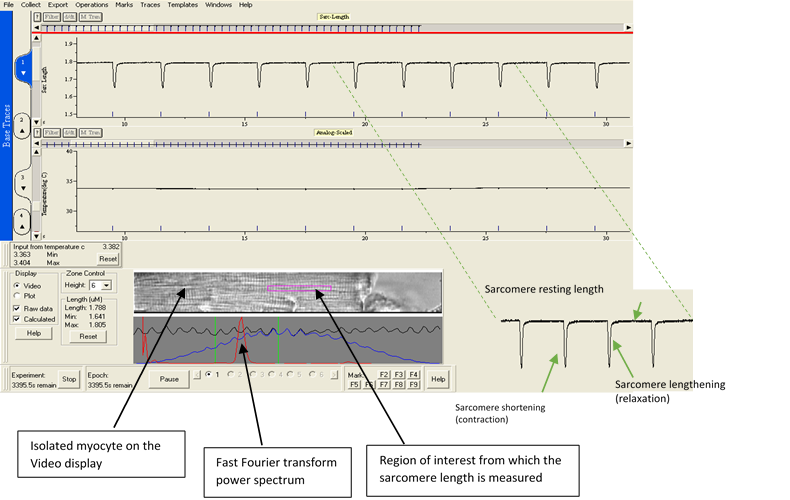
Myocardial contractility is an essential property of the normal heart. Drug-induced impairment of the regulatory machinery governing cardiac contractility can seriously affect cardiac function. Our contractility assay measures changes in sarcomere shortening of isolated ventricular myocytes in response to compounds, helping to evaluate their impact on cardiac function.
Why do we need to determine the effects of our compounds on hERG channels?
The human ether-a-go-go related gene (hERG) potassium channel is encoded by the KCNH2 gene and conducts the rapid delayed rectifier potassium current (IKr) in human cardiomyocytes. IKr is an important contributor to the repolarization phase of the cardiac action potential. Drug-induced inhibition of IKr increases the action potential duration (APD), resulting in prolonged QT interval on the surface electrocardiogram. QT prolongation can lead to Torsades de Pointe (TdP) that can degenerate into ventricular fibrillation and sudden cardiac death. Importantly, hERG channels are particularly prone to block by compounds with diverse chemical structures because of unique structural properties: 1) A large volume of the pore inner vestibule which favors drug entry, and 2) the presence of two aromatic residues in the S6 domain promoting binding with drugs containing aromatic moieties through pie electron stacking.
Aurora can measure drug-induced inhibition of hERG channels using the rubidium flux assay or manual patch clamp.
Our compound inhibits hERG channels in vitro. Should we terminate it?
Not yet! Although hERG channel inhibition may result in an increase in action potential duration (APD) and QT interval prolongation, not all hERG channel inhibitors induce torsades de pointes (TdP). In fact, it is now recognized that QT interval prolongation alone is not sufficient to predict proarrhythmic risk. For example, verapamil which is a potent hERG blocker is used therapeutically. Verapamil does not produce TdP because it simultaneously inhibits the L-type Ca2+ current (ICa,L), offsetting the effect of hERG block. Generally, drugs inhibiting inward currents such as the late sodium current (INa,L) and ICa,L in addition to IKr (or even other potassium channels like IKs) are less likely to induce arrhythmias and can even be antiarrhythmic.
In the context of the E14/S7B Q&As (February 2022), under a scenario where a positive hERG signal is combined with prolonged QT in vivo, additional studies can be performed to further explore the risk of developing arrhythmias.
Aurora has the expertise to guide you through this process and has the capability to test additional cardiac ion channels to complement the hERG assay.
What is the nonclinical strategy for assessing risk of delayed ventricular repolarization and development of Torsades de Pointe (TdP) arrhythmias?
A mechanistic understanding of the generation of torsades de pointe (TdP) and the development of new physiologically relevant assays have made it possible to obtain more information from nonclinical assays to predict TdP risk. Because the cardiac action potential results from the orchestrated opening and closing of multiple ion channels in a time- and voltage-dependent manner, an integrative approach is required. The in vitro hERG assay and in vivo QT assay, combined with additional cardiac ionic currents and cellular repolarization assays including the hiPSC-CM assay can be used for risk assessment of drug-induced proarrhythmia. As hERG block can be offset by inhibition of inward sodium or calcium currents, testing additional ion channels can therefore inform on the risk of developing TdP. To confirm potential effects on repolarization and risk of developing arrhythmias, drug-induced effects on action potentials recorded from hiPSC-CM can be determined.
Using multiple cellular assays, Aurora complies with the application of the 3Rs (replacement/reduction/refinement) principles in the design of a nonclinical integrated risk assessment strategy.
What is the impact of nonclinical data on the clinical program?
To date, nonclinical studies as described in ICH S7B have primarily inform investigators on the safety profile of their compounds before entering clinical development but have not been generally considered in the regulatory decision-making process once drugs enter clinical studies. An integrated risk assessment allows the use of nonclinical data to reduce the need for thorough QT (TQT) studies.
For example, in a scenario where nonclinical studies conclude that a drug is at low risk for QTc interval prolongation at exposures exceeding the high clinical exposure prediction, clinical and nonclinical assessments can be used as a substitute for a thorough QT (TQT) study (Source: ICH E14/S7B Q&As Training material).


