PRODUCT INFORMATION
FlowFlex
COVID-19 Antigen Rapid Test
Each Kit Contains:
| 5-pack | 25-pack |
| 5 Test cassettes | 25 Test cassettes |
| 5 Extraction buffer tube | 25 Extraction buffer tube |
| 5 Disposable nasal swab | 25 Disposable nasal swab |
| 1 Product insert | 25 Tube stand |
| 1 Product insert |
Specification:
- Sample Type: Nasal and Nasopharyngeal Swabs
- Detection Method: lateral flow chromatographic immunoassay
- Detection Time: 15-30 minutes
- Relative Sensitivity: 97.1% for Nasal; 97.6% for Nasopharyngeal
- Relative Specificity: 99.5% for Nasal; 99.4% for Nasopharyngeal
- Accuracy: 98.8% for Nasal; 98.7% for Nasopharyngeal
- Health Canada Approved under Interim Order (# 327573)
- Shelf Life: 24 months
CovClear (2/25-Pack) – Home OTC
COVID-19 Antigen Rapid Test
Each Kit Contains:
- 2/25 Lateral flow assay strips
- 2/25 Chase buffer ampules
- 2/25 Vials
- 2/25 Locking caps
- 2/25 Individually wrapped swabs
- 2/25 Instructions for use
- 2/25 Quick reference guides
Specification:
- Sample Type: Nasal swab
- Detection Time: 20 minutes
- Sensitivity/PPA: 95.5 %
- Specificity/NPA: 100 %
- Manufactured in the United States
- Interim order #330172
- Materials required but not provided: a pair of gloves and timer
Standard Q
COVID-19 Antigen Rapid Test
Each Kit Contains:
- 25 Test devices
- 25 Extraction buffer tubes
- 25 Nozzle caps
- 25 Sterile swabs
- 1 STANDARD COVID-19 Ag Positive Control swab
- 1 STANDARD Respiratory Negative Control Swab
- 2 Buffer tube racks
- 1 Instructions for use
Specification:
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 15-30 minutes
- Relative Sensitivity: 97.12% for nasal swab
- Relative Specificity: 100 % for nasal swab
- Interim order: #328889
- All necessary reagents provided & no equipment needed
Artron
COVID-19 Antigen Rapid Test
Each Kit Contains:
- 25 Test cassettes with desiccant in individual pouch
- 25 Extraction tubes sealed with sample extraction buffer (300µL/tube)
- 25 Extraction tube caps
- 25 Nasal swabs
- 1 Tube rack
- 1 Instruction for use
Specification:
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 20 minutes
- Relative Sensitivity: 91.3 %
- Relative Specificity: 99.56 %
- Interim order # 327866
- All necessary reagents provided & no equipment needed
Rapid Response®
COVID-19 Antigen Rapid Test
Each Kit Contains:
| 5-pack | 25-pack |
| 5 Test cassettes | 25 Test cassettes |
| 5 Individually packed swabs | 25 Individually packed swabs |
| 5 Individually packed buffers | 2 Bottles of extra buffer liquid |
| 5 Tubes and nozzles | 25 Tube and Nozzles |
| 1 Tube stand | 1 Tube stand |
| 1 Product insert | 1 Product insert |
Specification:
- Sample Type: Nasal and Nasopharyngeal swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
- Relative Sensitivity: 95.60 % for NP swab; 90.20% for nasal swab
- Relative Specificity: 100 % for NP swab; 100% for nasal swab
- Interim order: #321669
- All necessary reagents provided & no equipment needed
Boson
COVID-19 Antigen Rapid Test

*Application: lab-based only
Each Kit Contains:
- 20 Individually packed test cards
- 2 sample buffers (4mL/each)
- 20 Individually packed sterilized swabs
- 20 Individually packed extraction tubes
- 1 Tube rack
- 1 Instruction for use
Specification:
- Sample Type: Nasopharyngeal swab
- Detection Method: Colloidal Gold
- Detection Time: 15-20 minutes
- Relative Sensitivity: 93.75 %
- Relative Specificity: 98.04 %
- Interim order #323096
QuickVue At-Home OTC (2/25-pack)
COVID-19 Antigen Rapid Test

Each Kit Contains:
- 2/25 Individually wrapped sterile foam swabs
- 2/25 Individually packed single-use strips
- 2/25 Pre-filled tubes
- 1 Tube holder
- 2/25 Nasal swabs
- 1 Instruction Sheet
- 1 Fact sheet for individuals
Specification:
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
- Interim order #331681
Resources
Product Insert (pdf)
Health Canada Authorization Letter (pdf)
Training video
FaStep
COVID-19 Antigen Rapid Test
Each Kit Contains:
20 Test cassettes, 20 Individually packed swabs, 20 Extraction buffers, 1 Tube stand, 1 Product insert
Specification:
- Sample Type: Nasal and Nasopharyngeal swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
- Relative Sensitivity: 94.3 % (84.6% ~ 98.1%)
- Relative Specificity: 98.3 % (95.1% ~ 99.4%)
- Overall Agreement: 97.4 % (94.4% ~ 98.48%)
- Interim order: #321478
- CE certified
- No additional instrument required to read the results
iStatis
COVID-19 Antigen Rapid Test

Each Kit Contains:
- 1 Test Cartridge
- 1 Nasal Swab
- 1 Vial Holder
- 1 Buffer Vial
- 1 Visual Card
- 1 Buffer Vial Cap
- 1 Package Insert
Specification:
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
- Relative Sensitivity: 100%
- Relative Specificity: 100%
- Interim order: 344422
PCL(2-pack)
Self Test COVID-19

Each Kit Contains:
- 2 test cards
- 2 extraction buffer tubes
- 2 filter caps
- 2 funnel applicators
Specification:
- Sample Type: Saliva
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
- Relative Sensitivity: 91 %
- Relative Specificity: 99 %
- Interim order #341676
Resources
Product Insert (pdf)
Health Canada Authorization Letter (pdf)
Training video
SGTi-flex(5/25-pack)
COVID-19 Antigen Rapid Test
Each Kit Contains:
- 5/25 single use test devices individually foil pouched
- 2 bottles of extraction solution
- 5/25 single use extraction tubes
- 5/25 single use dropper tips
- 5/25 sterilized anterior nasal (and nasopharyngeal) swab
- 1 Instruction Sheet
- 1 Fact sheet for individuals
Specification:
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
- Relative Sensitivity: 95.7%
- Relative Specificity: 99.38%
- Interim order #321979
Resources
Product Insert (pdf)
Health Canada Authorization Letter (pdf)
Training video
Not sure which test to order? Read the product comparison review by our product specialists and choose the antigen test that best suits you.
The COVID-19 Antigen Rapid Test Device is a lateral flow in-vitro immunoassay intended to be used for the qualitative detection of the SARS-CoV-2 viral nucleoprotein. These devices differ in sample collection method, packaging, and sensitivity. Please speak to our product specialist to determine which one is right for your organization.
Results and Interpretation
Want more detail on how to interpret the Canada COVID-19 Antigen Rapid Test? See our article here!
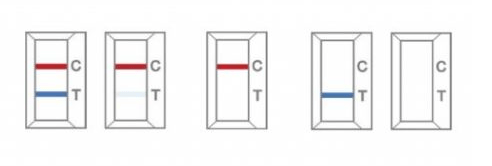
POSITIVE: Both the Antigen test line (T) and the control line (C) are colored in the COVID-19 Antigen Rapid Test Cassette.
NEGATIVE: One coloured line appears in the control region. No apparent coloured line appears in the Antigen test region.
INVALID: Control line fails to appear. Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette.
NOTE: The colour intensity in the test region (T) may vary depending on the concentration of analytes present in the specimen. Therefore, any shade of color in the test region should be considered positive.
Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
Limitations
- This test is recommended for Healthcare professional use only.
- The test procedure, precautions and interpretation of results for this test must be followed strictly when testing. Failure to follow the Test Procedure and Interpretations of Test Results may adversely affect test performance and/or invalidate the Test Result.
- The test should be used for the qualitative detection of SARS-CoV-2 antigen in human nasopharyngeal swab specimens. Neither the quantitative value nor the rate of SARS-CoV-2 antigen concentration can be determined by this qualitative test.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Positive test results do not rule out co-infections with other pathogens.
- False-negative test results are more likely during peak activity when prevalence of disease is high.
- False-positive test results are more likely during periods of low SARS-CoV-2 activity when prevalence is moderate to low.
Frequently Asked Questions
The regulations around COVID testing for travel is highly dependent on the country you are traveling to. While rapid antigen tests may be accepted in some countries, these tests need to be performed and signed-off by a trained individual, doctor, or pharmacist.
Pursuant to Section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on February 18, 2020, The FaStep Rapid COVID-19 IgG/IgM Rapid Test Device and Rapid COVID-19 Antigen Test (both distributed by Aurora) are now authorized for sale or importation in Canada.
No, these rapid tests do not need any additional instruments to read the results. These test kits are self-contained, containing all the tools you need from sample collection to result reading.
This test has been designed to act as a supplementary test for suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. Results from nucleocapsid protein antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.
We carry many brands of rapid antigen kits and some are authorized for at-home testing or self-testing while others are for use by trained laboratory or healthcare professionals. Please check the product description for details. Laboratories are required to report all results to the appropriate public health authorities.
Yes! Aurora Biomed Inc. holds a Medical Device Establishment License (MDEL #17977) from Health Canada for the importation of Class 4 medical devices. COVID-19 Rapid Antigen kits fall under Class 4 certification.
Please interpret the result of rapid antigen and antibody tests within 15-30 minutes. The result is not valid if you check again in a few hours.