
PRODUCT INFORMATION
Coronavirus (COVID-19) Antigen Rapid Test Cassette (OTC)
FDA has recently approved COVID-19 Rapid Antigen Test for OTC use under the Emergency Use Act. These COVID-19 Rapid Antigen test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests. This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals
CareStart
COVID-19 Rapid Antigen Home Test

Each Kit Contains:
- 2 Test devices
- 2 Sterile swabs
- 2 Extraction vials & caps
- 1 Individual factsheet
- 1 Quick start guide
Specification:
- PPA: 87%, NPA: 98%
- Sample Type: anterior nasal swabs
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
Key Features:
- FDA EUA Authorized
- Non-Invasive
- Detects all known COVID-variants
- Simple to use
- No additional instrument required to read the results
- Cost-efficient
Packaging Configuration:
- 2 tests per kit, 464 tests per case, 9,280 tests per pallet
FlowFlex
COVID-19 Antigen Home Test
Each Kit Contains:
- 1 Test cassette
- 1 Extraction buffer tube
- 1 Disposable nasal swab
- 1 Package insert
Specification:
- PPA: 93%, NPA: 100%
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
Key Features:
- FDA EUA Approved – OTC Use
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 1 test per kit, 300 tests per case, 6,000 tests per pallet
iHealth
COVID-19 Antigen Rapid Test
Each Kit Contains:
- 2 COVID-19 Test cards
- 2 Nasal swabs
- 2 Pre-filled or empty tubes with sealed solutions
- 1 Package insert
Specification:
- PPA: 94.3%, NPA: 95%
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 15 minutes
Key Features:
- FDA EUA Approved – OTC Use
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 2 tests per kit, 180 tests per case, 15,120 tests per pallet
QuickVue
COVID-19 Antigen Rapid Test – OTC Use

Each Kit Contains:
- 2 Test cassettes
- 2 Sterile swabs
- 2 Extraction tubes
- 2 Buffers
- 1 Package insert
Specification:
- PPA: 96.6%, NPA: 99.3%
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
Key Features:
- FDA EUA Approved – OTC Use
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 2 tests per kit, 180 tests per case, 2,700 tests per pallet
BinaxNow
COVID-19 Antigen Self Test

Each Kit Contains:
- 2 Test cassettes
- 2 Sterile swabs
- 2 Extraction tubes
- 2 Buffers
- 1 Package insert
Specification:
- PPA: 96.6%, NPA: 99.3%
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 10 minutes
Key Features:
- FDA EUA Approved – OTC Use
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 2 tests per kit, 12 tests per case, 1,728 tests per pallet
INDICAID
COVID-19 Antigen At-Home Test
Each Kit Contains:
Sold in 12pk or 2pk configuration
• 2/12 test devices
• 2/12 buffer solution vials
• 2/12 sterile nasal swabs
• Instructions for use
• Quick reference guid
Specification:
- 81.7% Sensitivity
- 99.4% Specificity
- Sample Type: Nasal swab
- Detection Method: Colloidal Gold
- Detection Time: 20 minutes
Key Features:
- FDA EUA Approved – OTC Use
- Non-Invasive
- Simple to use
- No additional instrument required to read the results
- Accessible, portable, and scalable option for COVID-19 testing
- Cost-efficient
Packaging Configuration:
- 2 Pack Configuration
Kit: 2 tests / 0.1 lb. / 8″ x 1″ x 2.6″
Case: 252 tests / 126 kits / 16.3 lbs. / 22.4″ x 16.9″ x 8.7″
Pallet: 6,048 tests / 3,024 kits / 389 lbs. / 40″ x 40″ x 58″ - 12 Pack Configuration
Kit: 12 tests / 0.4 lb. / 8.1″ x 3.6″ 2.6″
Case: 432 tests / 36 kits / 16.3 lbs. / 22.4″ x 16.9″ x 8.7″
Pallet: 10,368 tests / 864 kits / 40″ x 40″ x 58″
OHC
COVID-19 Antigen Self Test

Each Kit Contains:
- 2 Test cassettes
- 2 Sterile nasal swabs
- 2 Extraction buffer tubes
- 2 Tube filter caps
- 1 User manual quick guide
- 1 box (tube holder)
FEATURES & BENEFITS:
- Detects both Omicron and Delta variants
- Children starting at the age of 14 can self test. Children from ages 2 to 13 require the help of an adult
- Uses minimally invasive shallow nasal swab
- Easy visual read
- Fast results in ~ 15 minutes
- Relative Sensitivity: 91% ( 95% CI: 82.8 to 95.6%); Relative Specificity: 99% (95% CI: 95.2 to 99.6%)*
- Room temperature storage
Packaging Configuration:
- 2 tests per kit
How Does COVID-19 Antigen Rapid Test Cassette Work?
The COVID-19 antigen rapid test is for the detection of SARS-CoV-2 nucleocapsid protein antigen, detectable in upper respiratory specimens during the acute phase of infection. Positive results indicate the presence of viral antigens.
COVID-19 Antigen Rapid Test Cassette Instructions for Use*
1. Sample Collection
Anterior Nasal swabs
- Remove a nasal swab from the pouch.
- Insert the swab into one of patient’s nostrils up to 1 inch from the edge of the nostril.
- Slowly roll the swab 5 times over the surface of the nostril. Using the same swab, repeat this collection process in the other nostril. Take
approximately 15 seconds to collect the specimen. - Slowly remove the swab from the nostril while rotating it.
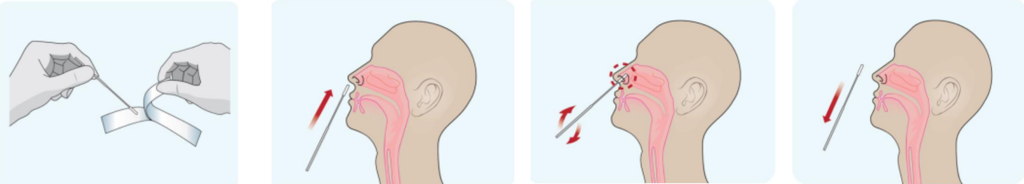
2. Sample Preparation
- Peel off aluminum foil seal from the top of the extraction vial containing the extraction buffer.
- Place the swab into the extraction vial. Rotate the swab vigorously at least 5 times.
- Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Properly discard the swab.
- Close the vial with the provided cap and push firmly onto the vial.
- Mix thoroughly by flicking the bottom of the tube.
*Disclaimer: Images above are for representation only. For kit-specific details regarding sample collection, preparation and detection, please read the instructions on the product insert inside each product
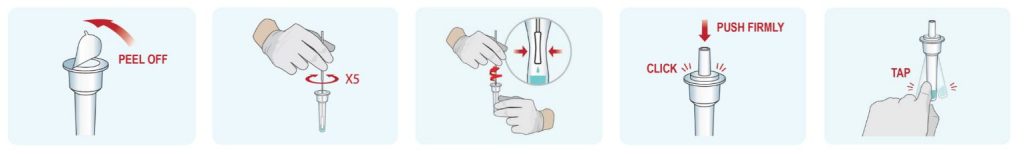
3. Detection
- Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well. 2 drops of the sample are required minimum volume to initiate the test run and invalid results will be obtained if 1 drop of sample is added to the cassette. Leakage of the sample is possible when 6 drops or more of the sample are added.
- Read and interpret the test result at 10 minutes. The test result should not be read and interpreted after 15 minutes.
*Disclaimer: Images above are for representation only. For kit-specific details regarding sample collection, preparation and detection, please read the instructions on the product insert inside each product
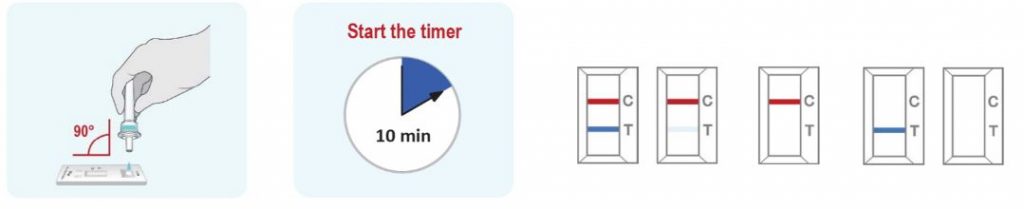
Interpretation of Test Results
For more detail about interpreting COVID-19 Antigen Rapid Test Results, see our article on this here.
**Disclaimer: Please see the image from Detection (step 3) of the ‘Instructions for Use’ section. The picture is for representation only, markings on test cassette may vary.
POSITIVE: Both the Antigen test line (T) and the control line (C) are colored in the COVID-19 Antigen Rapid Test Cassette.
NEGATIVE: One coloured line appears in the control region. No apparent coloured line appears in the Antigen test region.
INVALID: Control line fails to appear. Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the COVID-19 rapid test kit immediately and contact your local distributor.
Limitation of Coronavirus Antigen Rapid Test
- This test is recommended for Healthcare professional use only.
- The test procedure, precautions and interpretation of results for this test must be followed strictly when testing. Failure to follow the Test Procedure and Interpretations of Test Results may adversely affect test performance and/or invalidate the Test Result.
- The test should be used for the qualitative detection of SARS-CoV-2 antigen in human nasopharyngeal swab specimens. Neither the quantitative value nor the rate of SARS-CoV-2 antigen concentration can be determined by this qualitative test.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Positive test results do not rule out co-infections with other pathogens.
- False-negative test results are more likely during peak activity when prevalence of disease is high.
- False-positive test results are more likely during periods of low SARS-CoV-2 activity when prevalence is moderate to low.
Frequently Asked Questions (FAQs)
This product is approved by FDA under Emergency Use Authorization (EUA).
This test has been designed to act as a supplementary test for suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. Results from nucleocapsid protein antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.
No, the Rapid Antigen test does not require any other equipment. This test contains all the tools you need from sample collection to result reading.
The COVID-19 Rapid Antigen tests are not intended for at home use. These tests are recommended for healthcare professional use only. If you feel you need to be tested, please reach out to a local clinic or physician and ask them about offering this test.
Yes, this product is now available in Canada.
Please interpret the result of rapid antigen and antibody tests within 15-30 minutes. The result is not valid if you check again in a few hours.


