
PRODUCT INFORMATION
COVID-19 is an infectious disease caused by a new coronavirus that can spread from person to person. COVID-19 symptoms can range from mild (or no symptoms) to severe illness. The Coronavirus (COVID-19) Antigen Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 virus in nasopharyngeal or Anterior Nasal swab specimens for diagnosis of COVID-19.
How Does COVID-19 Antigen Rapid Test Cassette Work?
The COVID-19 antigen rapid test is for the detection of SARS-CoV-2 nucleocapsid protein antigen, detectable in upper respiratory specimens during the acute phase of infection. Positive results indicate the presence of viral antigens.
COVID-19 Antigen Rapid Test Cassette Instructions for Use*
1. Sample Collection
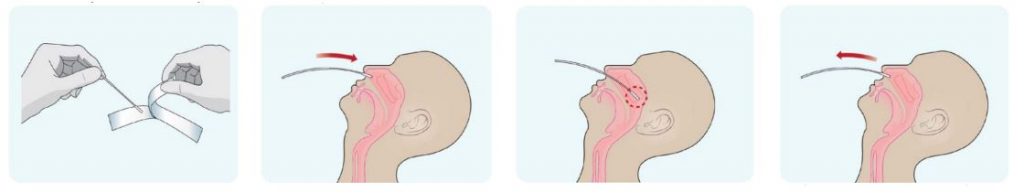
Nasopharyngeal swabs
- Remove a nasopharyngeal swab from the pouch.
- Place the swab into one of the patient’s nostrils until it reaches the posterior nasopharynx; keep insert until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient.
- Slowly rotate 3-5 times the swab over the surface of the posterior nasopharynx.
- Slowly remove the swab from the nostril while rotating it.
*Disclaimer: Images above are for representation only. For kit-specific details regarding sample collection, preparation and detection, please read the instructions on the product insert inside each product
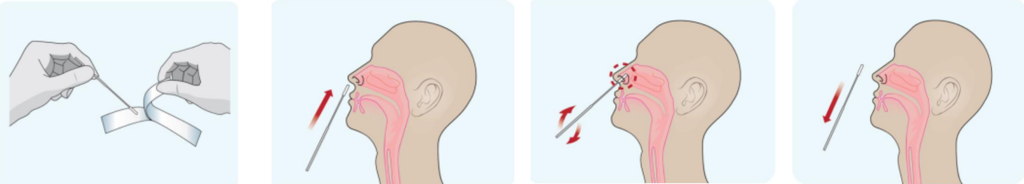
Anterior Nasal swabs
- Remove a nasal swab from the pouch.
- Insert the swab into one of patient’s nostrils up to 1 inch from the edge of the nostril.
- Slowly roll the swab 5 times over the surface of the nostril. Using the same swab, repeat this collection process in the other nostril. Take approximately 15 seconds to collect the specimen.
- Slowly remove the swab from the nostril while rotating it.
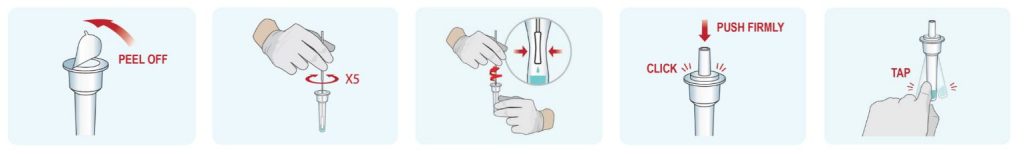
2. Sample Preparation
- Peel off aluminum foil seal from the top of the extraction vial containing the extraction buffer.
- Place the swab into the extraction vial. Rotate the swab vigorously at least 5 times.
- Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Properly discard the swab.
- Close the vial with the provided cap and push firmly onto the vial.
- Mix thoroughly by flicking the bottom of the tube.
*Disclaimer: Images above are for representation only. For kit-specific details regarding sample collection, preparation and detection, please read the instructions on the product insert inside each product
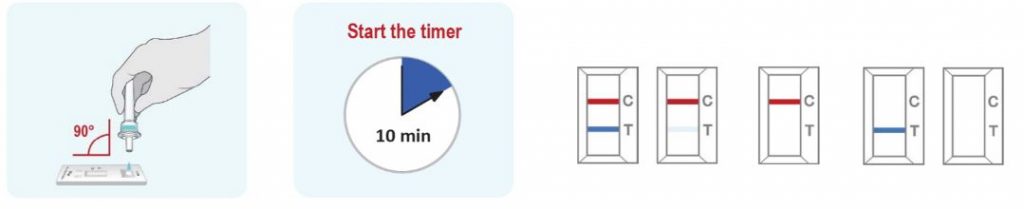
3. Detection
- Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well. 2 drops of the sample are required minimum volume to initiate the test run and invalid results will be obtained if 1 drop of sample is added to the cassette. Leakage of the sample is possible when 6 drops or more of the sample are added.
- Read and interpret the test result at 10 minutes. The test result should not be read and interpreted after 15 minutes.
*Disclaimer: Images above are for representation only. For kit-specific details regarding sample collection, preparation and detection, please read the instructions on the product insert inside each product
Interpretation of Test Results
For more detail about interpreting COVID-19 Antigen Rapid Test Results, see our article on this here.
***Disclaimer: Please see the image from Detection (step 3) of the ‘Instructions for Use’ section. The picture is for representation only, markings on test cassette may vary.
POSITIVE: Both the Antigen test line (T) and the control line (C) are colored in the COVID-19 Antigen Rapid Test Cassette.
NEGATIVE: One coloured line appears in the control region. No apparent coloured line appears in the Antigen test region.
INVALID: Control line fails to appear. Insufficient sample volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the COVID-19 rapid test kit immediately and contact your local distributor.
Limitation of Coronavirus Antigen Rapid Test
- This test is recommended for Healthcare professional use only.
- The test procedure, precautions and interpretation of results for this test must be followed strictly when testing. Failure to follow the Test Procedure and Interpretations of Test Results may adversely affect test performance and/or invalidate the Test Result.
- The test should be used for the qualitative detection of SARS-CoV-2 antigen in human nasopharyngeal swab specimens. Neither the quantitative value nor the rate of SARS-CoV-2 antigen concentration can be determined by this qualitative test.
- A negative test result may occur if the level of antigen in a sample is below the detection limit of the test.
- Positive test results do not differentiate between SARS-CoV and SARS-CoV-2.
- Positive test results do not rule out co-infections with other pathogens.
- False-negative test results are more likely during peak activity when prevalence of disease is high.
- False-positive test results are more likely during periods of low SARS-CoV-2 activity when prevalence is moderate to low.
Frequently Asked Questions (FAQs)
This product is approved by FDA under Emergency Use Authorization (EUA).
This test has been designed to act as a supplementary test for suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. Results from nucleocapsid protein antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.
No, the Rapid Antigen test does not require any other equipment. This test contains all the tools you need from sample collection to result reading.
The COVID-19 Rapid Antigen tests are not intended for at home use. These tests are recommended for healthcare professional use only. If you feel you need to be tested, please reach out to a local clinic or physician and ask them about offering this test.
Yes, this product is now available in Canada, please refer to the Health Canada Approved Rapid Test page for more information.
Please interpret the result of rapid antigen and antibody tests within 15-30 minutes. The result is not valid if you check again in a few hours.


