Imagine analyzing thousands of protein samples in a fraction of the time and with higher accuracy than ever before. With the power of automation in proteomics sample preparation for mass spectrometry (MS), this dream is a reality!
What is Proteomics?
Proteomics refers to the large-scale study of proteins within a biological system. It is the systematic identification and characterization of proteins for their structure, function, quantity, and molecular interaction. Utilizing proteomics techniques, numerous complicated biological materials, such as tissue, nipple aspirate fluid, cerebrospinal fluid, urine, blood, and saliva, have been characterized. The identified proteins were then followed up by matching with the peptide database.
Currently, the most popular platform for proteome investigations is Mass Spectrometry (MS). It is a method of analytical chemistry that assesses the abundance and mass-to-charge ratio of gas-phase ions to determine the number and type of molecules present in a sample. It is now possible to identify hundreds to thousands of potential protein candidates in a single experiment because of the development of high-resolution mass spectrometers.
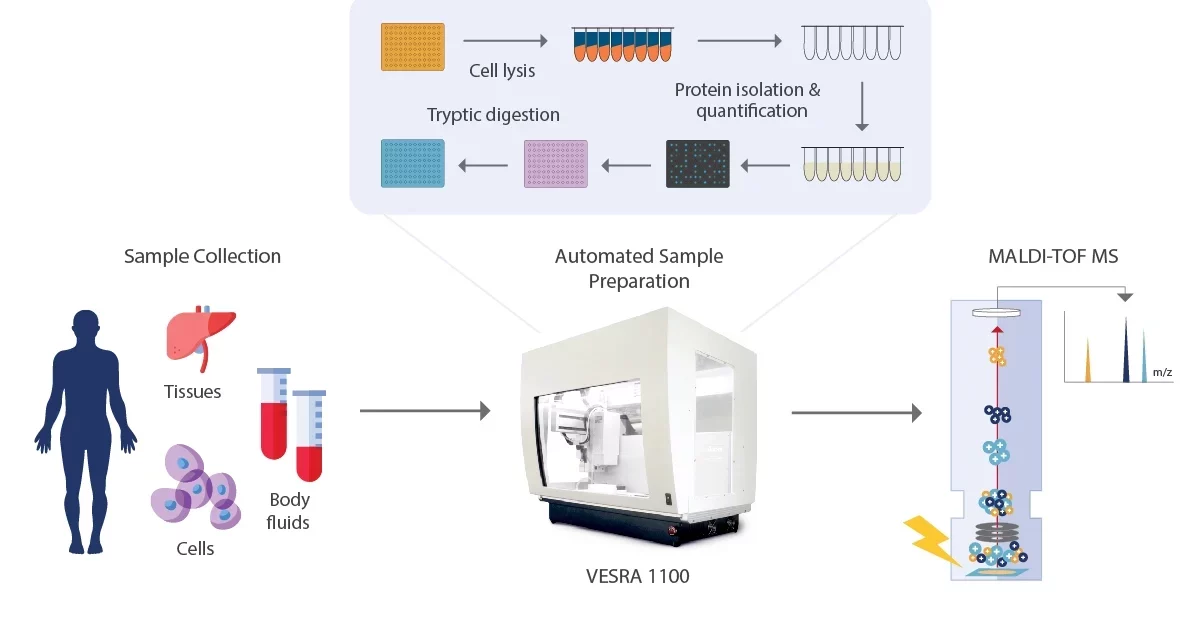
Higher throughput mass spectrometry is pivotal to managing the enormous growth in test samples currently observed in various clinical, forensic, and toxicological applications. The main bottleneck in mass spectrometry procedures is manual sample preparation which can be reduced by automation. Sample preparation is crucial as it improves the matrix’s suitability for analysis in several ways. The accelerated analytes obtained from a suitable matrix can remove any obstructive errors in the experiment). Some steps are involved in proteomics sample preparation, including protein extraction and protein separation.
Protein Extraction
The first step in proteomics sample preparation is to extract the proteins from the biological sample. This can be done using various methods, such as ultrasonication, homogenization, or lysis. The technique selected will depend on the sample type and the desired result. For example, homogenization may be used to break it down into smaller pieces if the sample is a tissue. Lysis may break open the cells if the sample is cell culture and release the proteins.
Protein Separation
The next step in proteomics sample preparation is to separate the proteins. This can be done using various methods, such as Gel electrophoresis, Chromatography, and Mass Spectrometry. The technique selected will depend on the sample type and the desired result. One of the most effective analytical methods is mass spectrometry, which is particularly effective since it can identify materials in very small concentrations (less than 100 picograms). By measuring the mass-to-charge ratio (m/z) of a molecule’s ion, a mass spectrometer may determine the molecule’s weight. Ions are created by forcing a neutral species to either lose or gain a charge. Ions are electrostatically driven into a mass analyzer after being created, where they are divided based on their m/z and then detected. To identify the components of the original sample, the outcomes of an MS experiment are typically compared to standards in a database determination, quantify known compounds and determine the structure and chemical properties of molecules which is a high level of analysis.
Automating the sample preparation process for proteomics analysis using mass spectrometry can involve liquid-handling robots to perform tasks such as protein extraction, digestion, and fractionation. This can significantly increase the efficiency and consistency of the sample preparation process, as well as reduce the risk of human error. Additionally, automation can increase the throughput of samples that can be processed, allowing for larger and more complex proteomics studies to be performed.
Some steps that can be automated include:
- Protein extraction from biological samples
- Digestion of proteins into peptides
- Purification of peptides
The VERSA Series offers different throughput possibilities, providing total flexibility and complete control over the entire instrument setup. Our innovative automated workstations include compact, cost-effective, and fully automated, high-throughput liquid handlers.
In conclusion, automating sample preparation for proteomics utilizing our VERSA series can significantly improve the analysis quality, reliability, and accuracy. It is possible to carry out operations like protein extraction, digestion, and fractionation more effectively and consistently.
Can this solution help your workflow?
Talk with one of our specialists and automate your lab right now.