Journal Club #1: MCU Mitochondrial Transporter
We all remember them from grad school: The dreaded journal club presentations where you were forced to present someone else’s work to a roomful of peers who are not necessarily interested in what you have to say. Nonetheless it forced us to pick up and read papers which we might not necessarily read, to look at the work and critically think about how the experiments were conducted, how the authors drew their conclusions, and what those conclusions might mean to the broader field in general. Journal club encouraged us as young scientists to ask critical questions of the research and even question the results of scientists more accomplished than ourselves in an effort to teach us best practices and improve our own research. In keeping with Aurora’s tradition of science, I have decided to post a bi-weekly “Journal Club” style series. Given that the Ion Channel Retreat is right around the corner, I figured I would kick things off by looking at an ion channel paper; given that this year we are expanding the ion channel retreat to include transporters, this paper focuses on a calcium transporter in mitochondria.
MICU1 and MICU2 Finely Tune the Mitochondrial Ca2+ Uniporter by Exerting Opposite Effects on MCU Activity
The paper I chose to focus on for this post is “MICU1 and MICU2 Finely Tune the Mitochondrial Ca2+ Uniporter by Exerting Opposite Effects on MCU Activity” published by Patron et al. in Molecular Cell earlier this year1. The authors examine the regulation of the Mitochondrial Calcium Uniporter (MCU) as several recent papers have described one regulatory sub-unit (MICU1) as both an activator2 and an inhibitor3,4 of MCU activity. Additionally, a homolog of MICU1, MICU2, was recently described5 leading the authors to further investigate how MCU is regulated.
Overall I found this study very well carried out. The authors used a mixture of genetic, immuno, and electrophysiology techniques to support their conclusions. Using western blotting combined with knock-down of MICU1 or MICU2 the authors found that the two proteins formed a heterodimer which interacted with MCU (MCU could be co-immunoprecipitated in pull down studies). This was further supported through co-localization studies using confocal microscopy and through FRET. The authors probed the roles of each of these proteins by producing knock-down conditions or over-expression conditions and monitoring the membrane potential at different concentrations of cytosolic calcium. The authors found that over-expression of MICU1 had a stimulatory effect on the migration of calcium across the lipid bilayer. Curiously, silencing of MICU1 also had a greater rate of calcium migration suggesting that MICU1 also plays an inhibitory role in calcium migration, keeping with what had been previously observed3,4. Curiously, when MICU1 was silenced the authors were also unable to detect any MICU2 within the cells suggesting that MICU1 is required for MICU2 protein stability (MICU2 mRNA was still observed in MICU1 silenced cells). When MICU1 was expressed with MICU2 silenced, the authors observed a similar stimulatory effect as when MICU1 alone was silenced, suggesting that MICU2 and not MICU1 was the true “gatekeeper” of MCU. The fact that no stimulation of channel activity was observed when calcium was not present indicated that calcium itself was acting as the signalling molecule.
Based on their findings the authors suggest a mechanism whereby at low cytosolic calcium concentrations MICU2 acts as a gatekeeper to keep MCU closed. Both MICU1 and MICU2 contain an EF-hand domain which could stimulate conformational change upon Ca2+ binding, as cytosolic calcium levels increase, MICU1 has its calcium binding sites occupied resulting in conformational change to keep MCU open, while calcium binding to MICU2 causes release of its inhibitory effect allowing mitochondrial accumulation of calcium.
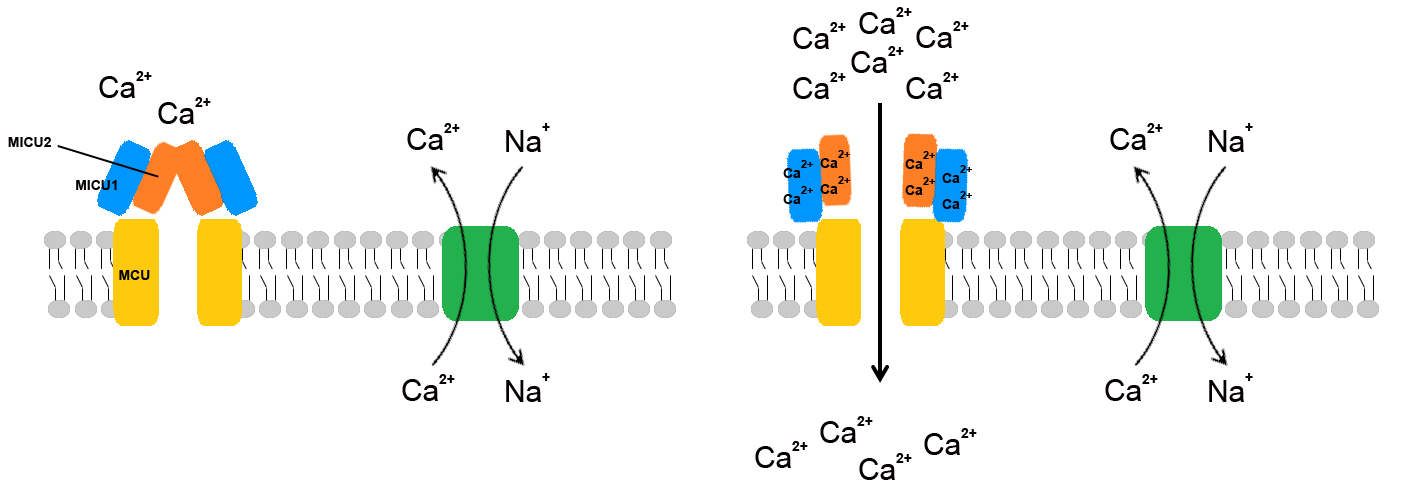
Although the authors suggest a conformational change due to calcium binding, the mechanism of such a change has yet to be elucidated, however, such a study is outside the scope of this report; conformational change upon calcium binding to an EF-hand domain has been well described, notably in the case of calmodulin. Also, the authors do not exclude the possibility of other regulatory proteins interacting with and modulating the MCU-MICU1-MICU2 complex; further studies should shed additional light on this interesting regulatory mechanism.
Calcium signalling plays an important role in our bodys physiology; generally during conditions where work is being performed, calcium is released into the cytosol resulting in mitochondrial uptake of calcium (which stimulates calcium sensitive dehydrogenases and upregulates ATP production). Loss of function of MICU1 has been linked to brain and muscle disorders6 as well as cardiac injury during reperfusion following an ischemic episode7. The role and mechanisms of calcium uptake in mitochondria with literature often finding confusing and sometimes contradictory results, we have only just begun understanding the roles that this important transporter plays.
What did you think of this paper? Feel free to share your thoughts or insights in the comments below or shoot us a tweet on Twitter @AuroraBiomed.
1. Patron M., Checchetto V., Raffaello A., Teardo E., Reane D.V., Mantoan M., Granatiero V., Szabo I., De Stefani D., Rizzuto R. (2014) MICU1 and MICU2 Finely Tune the Mitochondrial Ca2+Uniporter by Exerting Opposite Effects on MCU Activity. Mol Cell. 53(5): 726-737.
2. Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. (2010) MICU1 Encodes a Mitochondrial EF Hand Protein Required for Ca(2+) uptake. Nature. 467: 291-296.
3. Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G. (2013) MICU1 Controls Both the Threshold and Cooperative Activation of the Mitochondrial Ca2+ Uniporter. Cell Metab. 17: 976-987.
4. Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M.. (2012) MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 151: 630-644.
5. Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. (2013) MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE. 8: e55785.
6. Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, Bonthron DT, Pysden KA, Whyte T, Munteanu I, Foley AR, Wheway G, Szymanska K, Natarajan S, Abdelhamed ZA, Morgan JE, Roper H, Santen GW, Niks EH, van der Pol WL, Lindhout D, Raffaello A, De Stefani D, den Dunnen JT, Sun Y, Ginjaar I, Sewry CA, Hurles M, Rizzuto R; UK10K Consortium, Duchen MR, Muntoni F, Sheridan E. (2014) Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 46(2): 188-93.
7. Dunchen MR. (2004) Roles of Mitochondria in Health and Disease. Diabetes. 53(1): 96-102.


