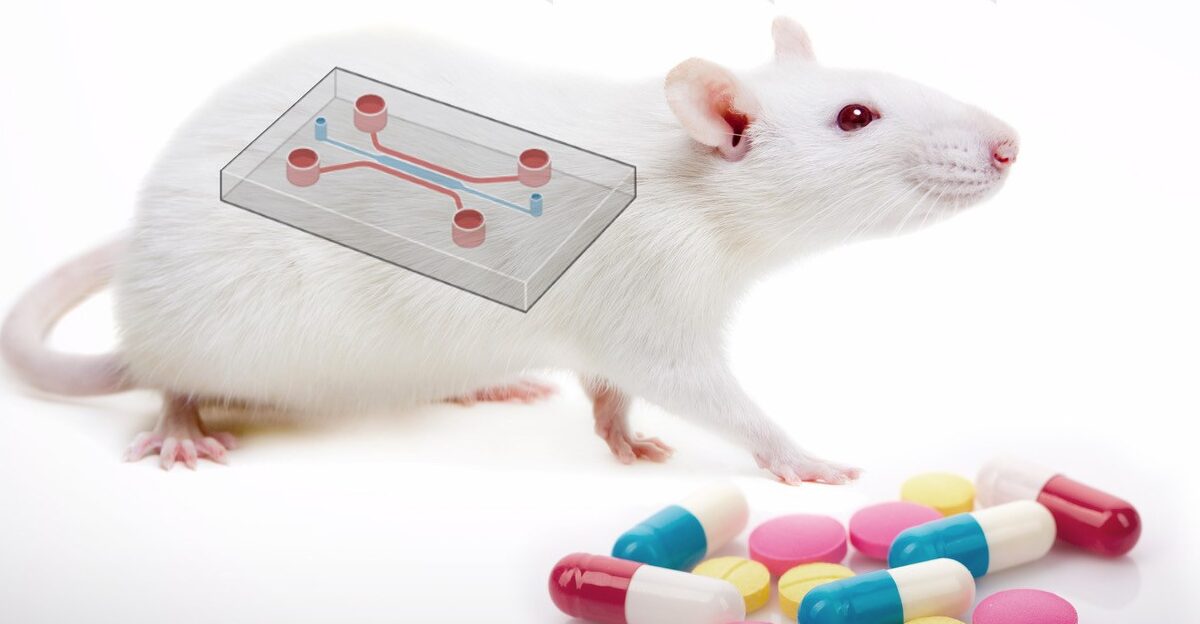
Development of safe and effective drugs for human use is a complex and time-consuming procedure that often involves animal testing. The use of animal models has been a traditional way to determine the safety and efficacy of a new drug before human clinical trials. However, animal testing is not always predictive of human physiology and has also been a source of ethical concerns.
To address the challenges of using animal models, the FDA Modernization Act 2.0 was enacted, which allows the drug development industry to use alternative models to animal studies before human clinical trials to determine the safety and efficacy of a new drug. This new act amends the Federal Food, Drug, and Cosmetic Act to clarify the methods sponsors can use to investigate the safety and efficacy of a drug.
Nonclinical tests are tests or studies that are used to predict human response based on scientific evidence and occur before or during the clinical trial phase of the investigation of the safety and effectiveness of a drug. These nonclinical tests may include cell-based assays, organ chips and micro-physiological systems, computer modeling, machine learning, and artificial intelligence.
The original act mandated that all drugs be tested on animals before being administered to humans for clinical trials.
To address challenges associated with animal testing, there is a pressing need for more human-translatable in vitro and in silico assays to be incorporated into preclinical workflows.
In a new era of precision medicine, such nonclinical tests may include:
- Cell-based assays (mammalian expression systems, human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM))
- Organ chips and micro-physiological systems: as an example, the FDA engaged in developing new technologies to assess drug safety and efficacy using human-based cells and tissues. The so-called “clinical trial in a dish” involves cells reprogrammed from patients (hiPSC) to test how a specific disease will respond to a new therapy. This approach applies to precision medicine, disease modeling, and drug testing. Ideally, testing drugs on patient-specific cells cultured in a dish will predict if the new drug benefits or harms the patient. A step further is to use 3D structures to mimic the properties of human tissue better. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5379998/
- Another alternative to animal testing is computer modeling, which can predict the efficacy and safety of drugs in silico. Powerful computer models can predict novel sites of binding for drugs to make them more effective and safer. These models can potentially significantly reduce the need for animal testing in drug development.
- Animal tests: Animal testing remains a useful tool to predict efficacy and toxicity. But emerging technologies help replace, reduce, and refine (3Rs).
It is important to note that the FDA Modernization Act 2.0 does not eliminate animal testing or state that it is unnecessary. Instead, it simply clarifies the definition of a nonclinical test or study under the Food, Drug, and Cosmetics Act to include additional and complementary testing methods. Animal testing remains an essential tool for predicting the complex physiological, neuroanatomical, reproductive, developmental, and cognitive effects of drugs to assess their safety and effectiveness before clinical trials and market approval.
In conclusion, the FDA Modernization Act 2.0 marks an essential shift in the drug development landscape as it allows for the use of alternative models to animal studies before human clinical trials to determine the safety and efficacy of a new drug. This new act provides drug development companies with a broader range of tools to investigate the safety and efficacy of a drug, while also addressing ethical concerns associated with animal testing. Using a combination of nonclinical tests, researchers and pharmaceutical companies can work towards developing safer and more effective drugs for human use.
One such example is use of high throughput screening (HTS) technologies like: Automated liquid handling systems and Label-free detection technologies that has greatly accelerated the pace of drug discovery and other scientific fields, enabling researchers to identify promising compounds much more quickly and efficiently than ever before.
Want to learn more?
Contact one of our specialists and understand how our solutions can help you in your research!