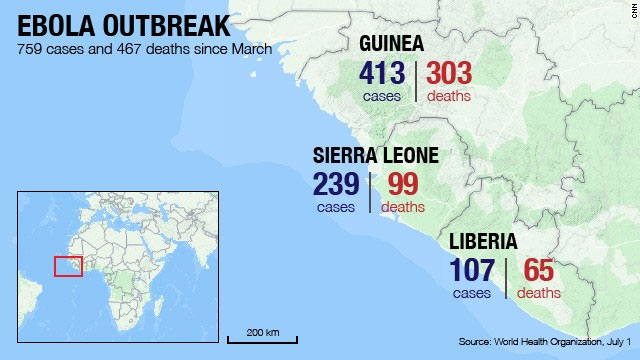
The current Ebola virus disease (EVD) outbreak is by far the largest and deadliest in history since the discovery of the virus in 1976. Four countries in Western Africa (Guinea, Liberia, Nigeria, and Sierra Leone) are facing the outbreak and are focusing their efforts on public health measures to control the outbreak. Since the beginning of the outbreak there has been a total of approximately 1800 cases of EVD in West Africa resulting in approximately 1000 deaths causing the WHO to declare the current outbreak as an international emergency.
EVD is a severe and potentially deadly acute viral illness often characterized by the sudden onset of fever, intense weakness, muscle pain, headache and sore throat. This is followed by vomiting, diarrhoea, rash, impaired kidney and liver function, and in some cases, both internal and external bleeding. The virus is spread through contact with infectious bodily fluids, unlike other well known viruses like Influenza and SARS which are airborne diseases. People are infectious as long as their body fluids contain the virus, even after death. The incubation period of the virus (time from initial infection to onset of symptoms) ranges from a couple days to 3 weeks.
The WHO was first notified of the outbreak by The Ministry of Health of Guinea in March and as of August 4th 2014, the cumulative number of cases attributed to EVD in Sierra Leone, Liberia, Guinea and Nigeria stands at a 55% mortality rate. Regrettably health authorities in the affected countries are unable to control the outbreak, and only experimental drugs are available for which safety and efficacy in humans has not been assessed. Some of these drugs have been successfully been tested in monkeys but not in humans.
On August 5th, the US FDA issued an Emergency Use Authorization (EUA) to authorize the emergency use of the U.S. Department of Defense Real-time RT-PCR Assay for the presumptive detection of Ebola Zaire virus in inactivated specimens from individuals in affected areas with signs and symptoms of the disease or who are at risk for exposure or may have been exposed to the virus. On August 6th, the U.S. FDA enabled the potential use of an experimental drug (the only one currently under phase 1 clinical trial) manufactured by a Canadian pharmaceutical company based in Burnaby to treat infected patients with EVD.
Rapid test kit is key in identifying the disease and isolating potentially infected individuals. Staying informed about news and current public health situations is important. Sample processing times can quickly become a bottle neck in cases such as this; Africa is simply not properly equipped to identify and contain this outbreak, however, it is unlikely to spread in North America due to a higher level of efficiency in identification and containment. To learn more about Ebola visit the WHO website.


